Setting the Global Standard for Accurate Detection of Antimicrobial Resistance.
Sensititre Instrumentation
Sensititre provides the only fully customizable system (ARIS 2X, SWIN, OptiRead, AIM, Vizion) with a choice of completely automated, semi-automated, or manual equipment to cover all diagnostic testing needs in the clinical laboratory.
More than 240 antimicrobials are available for susceptibility testing of bacteria and yeasts, ensuring accurate first time results.
Sensititre remains the system of choice for global surveillance programs, including NARMS (National Antimicrobial Resistance Monitoring System), coordinated via FDA-CVM, USDA, CDC.
Sensititre® Microbiology Systems
The Sensititre ARIS 2X is a fully automatic, bench-top incubating and reading system that reduces workload and speeds the laboratory routine. ARIS 2X fits onto the AutoReader and uses an internal barcode scanner to identify each plate type, assign the appropriate incubation time, and when this assigned time has elapsed, transport the plate to the AutoReader –with no manual intervention.
- 64-plate capacity allows user to load any MIC, breakpoint, or identification plates for a combination of 192 possible tests on a single instrument
- Heated carousel individually incubates all plates to ensure optimal growth conditions and elimination of test repeats
- Plate-specific barcodes provide automatic inventory to allow user to load or unload tests at any time, in any location

Sensititre ARIS® 2X

Sensititre ARIS® 2X SWIN® Software
Introducing ARIS 2X and SWIN The Leading Provider of “Gold Standard” Dry Susceptibility Products to Detect Antimicrobial Resistance
- Greatest number of FDA cleared antimicrobics, with the least number of restrictions, eliminates multiple offline test procedures, saving time and expense
- True MIC readings in accordance with NCCLS recommendations ensure accurate endpoint results, leading to better patient care
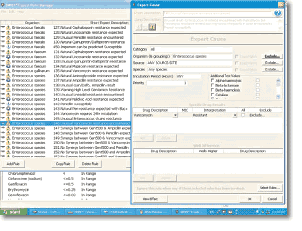
- Intelligent and customizable Expert System permits appropriate intervention for organisms with unusual resistance patterns
- Improved instrument automation with no "reagent additions" provides more consistent performance and requires less maintenance
- Unique, color coded identification parameters allow immediate recalculation of results, reducing labor and saving costs
- Excellent quality and consistent results lead pharmaceutical companies to choose Sensititre for surveillance studies and clinical trials
- Extended shelf life and room temperature storage simplify inventory control concerns and reduce storage and expiry costs
ARIS 2X AND SWIN ... THE WINNING COMBINATION IN THE FIGHT AGAINST ANTIMICROBIAL RESISTANCE.

Sensititre AIM™ Automated Inoculation Delivery System
Fast, accurate dosing mechanism eliminates skipped wells and costly repeat tests
- Compact modular design allows individual unit placement in microbiology safety cabinet or on laboratory bench
- Easy-to-use, icon-driven touch screen allows quick selection of dosing volumes and patterns
- Compatible with most 96 well microtitre plates to ensure flexibility with dry or frozen susceptibility formats
- Unique tube design and manually applied seal provide additional protection against biohazardous spill concerns

Sensititre® Windows Software SWIN®
Introducing SWIN Software from the Leading Provider of Dry Custom Susceptibility Products
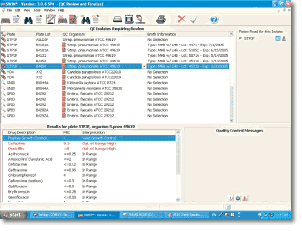
- Intelligent and customizable Expert System provides three-tiered expert messages: Information, Modification (based on CLSI recommendations), and Warning Levels
- Practical customization options enhance data entry and demographic options
- Quality control module allows technologist to easily manage susceptibility plates, identification tests, and broth information for inspection purposes
- Immediate alert to out of range results enhances workflow and automatic report function expedites release of results in range
- Drop down fields eliminate difficult codes and numbers
- View ID details and susceptibility results at the same time
- Manual, semi-automated, and automated read options available on single software platform
Review and Finalize QC Options
Customized Expert Rule Options